Do all liquids evaporate at the same rate variables on both sides
These factors are related to the nature of the liquid. Like we have observed here, liquid turning into gas because of a pressure drop negative pressure inside the pump head or in the suction-side piping is called cavitation. Beer is a mixture of water, alcohol, and carbon dioxide gas, but to make the explanation simple, we will use pure water.
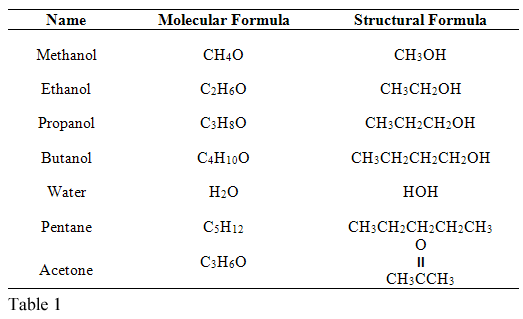
Examples include, methanol, acetone, and other low-molecular-weight organic solvents. Water molecules start moving more intensely in elevated temperatures. Beer is a mixture of water, alcohol, and carbon dioxide gas, but to make the explanation simple, we will use pure water. There are also other factors that can foster cavitation, aside from a through d above.
When the diaphragm of the diaphragm pump moves out, negative pressure develops inside the pump head. Vaporization of Liquid Evaporation. Next, we cover the container, sealing it completely as shown in Fig.

When the diaphragm of the diaphragm pump moves out, negative pressure develops inside the pump head. Occurs more easily with liquids that vaporize easily i. The example using beer in " Let's now consider the other way around. In this section, let's look at cases where the liquid itself turns into gas vaporization instead of dissolved gas escaping from the liquid.

Here, the force pressing down on the liquid surface gets weaker, which makes it easier for water to boil. Next, we cover the container, sealing it completely as shown in Fig. Water molecules are bonded to each other while the temperature is low, but when the temperature rises to a certain point, the bond breaks due to the increased molecular motion. As might be expected, the lower the pressure, the lower the boiling point will also be.
These factors are related to the nature of the liquid. In this section, let's look at cases where the liquid itself turns into gas vaporization instead of dissolved gas escaping from the liquid. We have already discussed in detail how inertial resistance occurs from pulsation of a diaphragm pump in "

We do this by removing the air inside the container using a vacuum pump. This temperature is called the boiling point. Like we have observed here, liquid turning into gas because of a pressure drop negative pressure inside the pump head or in the suction-side piping is called cavitation. We have already discussed in detail how inertial resistance occurs from pulsation of a diaphragm pump in " Water boils at degrees Centigrade.

Furthermore, water boiling inside the pump means that gas enters into the pump head, which significantly reduces the efficiency of the diaphragm pump. These factors are related to the nature of the liquid. Water molecules start moving more intensely in elevated temperatures.

As a result, rice can be cooked to perfection in a short time. There are also other factors that can foster cavitation, aside from a through d above. Water molecules start moving more intensely in elevated temperatures. This temperature is called the boiling point.

These factors are related to the nature of the liquid. Beer is a mixture of water, alcohol, and carbon dioxide gas, but to make the explanation simple, we will use pure water. Like we have observed here, liquid turning into gas because of a pressure drop negative pressure inside the pump head or in the suction-side piping is called cavitation. In summary, the factors that facilitate cavitation are, as in the case of inertial resistance: We have already discussed in detail how inertial resistance occurs from pulsation of a diaphragm pump in "